The concept of the chemical bond is well known to all from the chemistry course. It processed so fundamentally that any information that can change our understanding of chemical binding is a sensation. It is known that the length of chemical bonds is comparatively permanent and its great elongation is always followed by the molecular decomposition. By the employees of Department of Organic Chemistry and Technology of Organic Substances FCT Ph.D., researcher P. O. Hunchenko and researcher L .V. Chernysh involving student Ye. Yu. Tykhonchuk under the direction of Dr. Sc., prifessor A. A. Fokin, in collaboration with the universities of Giessen (Germany) and Stanford (USA) were challenged a nature. Using as starter compounds called diamondoids (hydrocarbons of nanometers size that reproduce fragments of natural diamond structures and produce from oil), they synthesized molecules containing an anomalous elongated bounding.
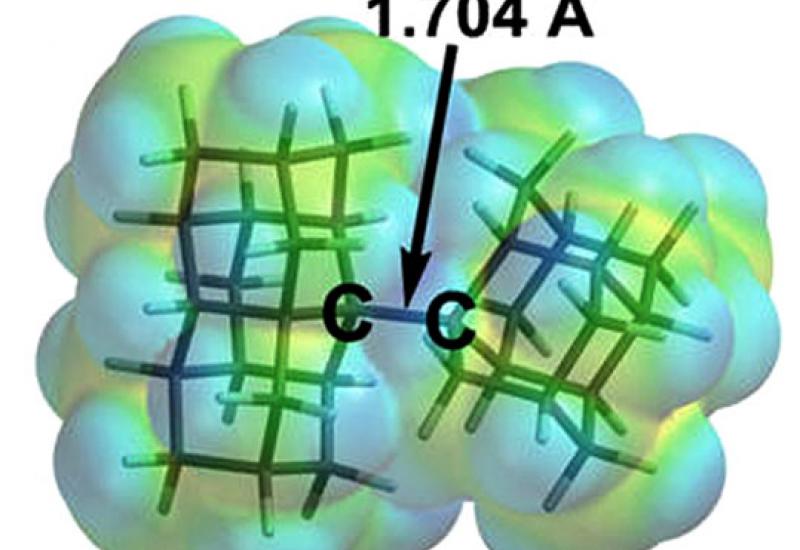
A new world record for the length of the carbon bond C-C currently is 1.704 Ao (typically 1,540 Ao). Remarkable is that despite on the record bounding length, synthesized compounds are stable even at elevated temperatures. The result was so unpredictable that it was necessary to undertake specific study causes of anomalous stability and this has led to fundamental discovery.
Early was considered that there are two different types of interactions between atoms: weak intermolecular (Van der Waals forces) and strong interatomic that were referred to the chemical binding. It was discovered that the compounds of ultra-long bonds that were received at the KPI stabilized not only by conventional forces, but also by weak Van der Waals interactions. The figure shows an example of a molecule with its own surfaces Van der Waals forces around ultra-long bond.
Therefore, firstly in the world experimentally was discovered that weak Van der Waals forces can have a decisive influence on the individual molecules’ stabilization. It offers the challenge of creating a material in which the strong bindings will equal in intensity to many weak binding which will be on the verge between individual molecule and nonmaterial. The results were so significant that were published in the authoritative scientific journal Nature (2011, vol. 477 p.308-311). It is worth noting that this is the first work of Ukrainian chemists published in this journal.