Recently, the Foucault pendulum, which is considered to be the fifth most important natural experiment that changed the world, was opened at our university. But what are the others? This is exactly what this publication is dedicated to.
However, something else at first: many of the advanced countries are so deeply aware of the role of new knowledge at the modern stage of development, that the UN started to declare one or the other year as years of science. Let us recall: 2005 was the year of Physics, 2009 - Year of Astronomy, and the current 2011 year is the year of Chemistry. Clearly, this indicates an increase of public interest in cognition as a process and understanding of the fact, that there is no future without science. Hopefully, that times, when at the school bench youth will show interest in scientific and educational spheres as a matter of life, will return. First of all I mean physics. Not even all the graduates whose speciality is physics are going to deal with this science, considering it complicated and immense. Therefore, in spite of the above, I’ll try to persuade those who are not sure, because the amount and depth of received and known facts in some cases scares even the most bright and competent.
I’ll start with the question: whether in physics you really have to know and remember everything that was gained by predecessors? Indeed, from a variety of popular and academic books we know a lot. Well, for example, that the Earth is not flat, as it seems, but spherical with a radius of about 6500 km. On the other side of the sizes, nuclei of atoms, never seen by a man before, which as we know are made up of protons and neutrons and their radius is tiny from the point of view of household, size 10-13 cm.
Gravitational and Coulomb interactions are fading inversely proportional to the square of the distances between the bodies or charges. In our galaxy there are about 1011 stars and the temperature on the surface of the Sun is estimated to be 6000 0C. These simple facts are just a small part of thousands of others, completely different. All of them together form an ordered mosaic for those who understand, which is called the physical world. However, even the well-known scientists can’t remember all historical data about the universe. What is more, there is no existing supercomputer, which can accommodate all the relevant data together.
It becomes clear that not only remember, but also write somewhere such amount of letters and numbers is impossible. Fortunately, you don’t have to do this! This is exactly what the incomprehensible harmony of nature is about, when an infinite variety of observed and principally allowed for the implementation of natural and man-made effects based on a finite and relatively small number of fundamental principles which are called laws. The main task of physics hasn’t changed – to form a unified theory, which would ideally contain a number of fundamental equations that describe all the known facts and would correctly predict new ones.
Inquisitive people are interested also in something else: how do we know and why are we so sure that all is going on exactly as physics says? For example, lets say that there are two protons and two neutrons in the nucleus of helium, that the Earth is spherical, that Maxwell's equations describe electromagnetic waves and so on. Everyone will answer – from the experiments, which mankind started to conduct long time ago, abandoning the simple contemplation of natural phenomena and replacing them with specially made, conscious laboratory experiments. It has long been when people made a conclusion that discovering nature may and have to be performed according to the scheme:
observed phenomenon - possible explanation - conclusions and predictions – laboratory -experiment - complete theory.
Indeed, after the observation of one or another process there comes a desire to explain it or express assumption of its causes; then come conclusions and analyzes of possible consequences, the test of which requires new experiments; if the prediction came true, the next is to form a more or less complete theory using the most modern mathematics and possible generalizations.
It seems simple and the chain of consistent actions is clear and executable. But it seems like that only at first glance, and there are examples when the time from the beginning to the end took a century. The most famous - the general structure of the universe, a scheme of which some thinkers began to offer long before BC, since it became a dominant of Ptolomey or geocentric system. Behind her, the center of the world was a fixed planet Earth and the Sun and other planets flew around. But under the pressure of further observations it all began to face serious difficulties when predicting the position of celestial bodies in the spherical sky were not consistent with observations. That's what made the Polish astronomer N. Copernicus in the middle of XVI century to abandon the geocentric model and put forward a fundamentally different one – heliocentric, according to which the Sun was elected as a center and the Earth was determined only as one of the planets, which have different orbits.
It was exactly an experiment that played a crucial role in its becoming.
During the years of science development a lot of experiments were conducted. It is impossible to tell about all of them, even with a strong desire. However, there are always experimentum cricis(lat) among of them - crucial, statement of which answered the deep questions of its time. Identifying them is not only quite complicated, it is a backbreaking task for anyone. However, appropriate selection can be made collectively, which for physical experiments actually was made during 2006 by one of the world's most famous newspapers New York Times, which conducted a survey of several thousand physicists. Each of them had to call the 10 most important experiments for the progress of past times. I consider it necessary to expand the erudition of KPI students and tell them about these experiments.
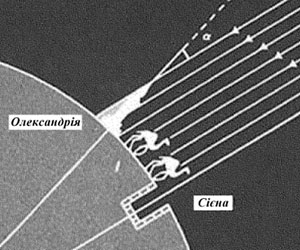 Eratosthenes Kirensk’s experiment.
Eratosthenes Kirensk’s experiment.
This ancient experiment was conducted in the third century BC, and it was dedicated to the measurement of the radius of the Earth. The scheme of the experiment is geniusly simple. At noon, the summer solstice, in Siena (now Aswan in Egypt), the Sun was in its zenith, and therefore objects didn’t cast the shadows. Similarly, in the same time in Alexandria, which is 800 km distant from Siena, Sun was rejected from the vertical by about 7o, which is about 0.02 of a full circle. From here you can easily calculate that the Earth's circumference is 40,000 miles, and its radius - 6300 km. It is amazing that such a simple way found radius of only 5% less than it is known today.
Experiment of Galileo Galilei
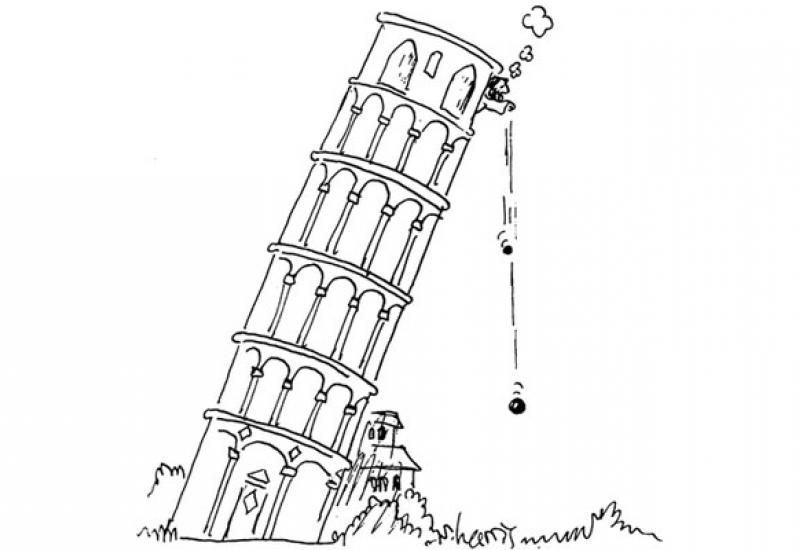
By the middle of XVII century the conclusion of Aristotle that the speed of the body fall depends on its weight, moreover heavier bodies fall faster, was considered to be correct. Based on this, Aristotle theorized that the Earth more attracts heavier bodies, and so they have to fall faster. In practice, not only gravity affects the fall, but also the air resistance.
Galileo decided to carefully check the ideas of Aristotle, for that he cast from the Leaning tower a cannon ball and a light musket ball. Since both objects had similar convex shape, the air resistance was almost the same for them. Thus, the researchers were able to determine that both bodies reach the earth simultaneously. In other words, it was experimentally or uniquely determined: the rate of fall does not depend on the mass, what has become a major factor for the development of mechanics.
Another experiment of Galileo Galilei
The top ten also hit the research on the measurement of the inert forces. Galileo measured the distances that rolling down an inclined plane balls crossed at regular intervals. When time was increased twice, the balls passed four times longer distance. This, in turn, testified that under the influence of gravity balls move rapidly. This conclusion was directly contrary to the well-known and such that people believed in for nearly two (!) Millenniums, which consisted in that under the influence of gravity, the bodies move with constant velocity, and if there is no gravity, they are motionless.
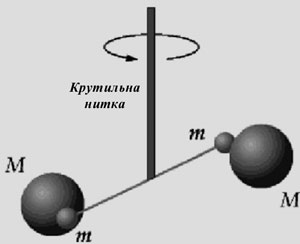 The Experiment of Henry Cavendish
The Experiment of Henry Cavendish
The law of universal gravitation states that the gravity between two bodies is directly proportional to their masses and inversely proportional to the square of the distance between them. But gravitational constant g was still unknown in this law. For its determination it was necessary to measure the attraction between two bodies with defined masses and known distance between them. This is a very difficult problem, because the corresponding force is very small. For example, we are in the gravitational field of the Earth, we feel it, but how exactly are we attracted with the filled dump truck, we do not know, because there is no impact on us.
Method of gravity measurement invited the fellow of Newton Henry Cavendish in 1798. He took spinning scales with two balls hanging on a thin light thread. Then the displacement of balls when approaching them with heavier items was measured. Thus, the scales reacted to disturbance, but due to the smallness it was difficult to measure it directly. Therefore, Cavendish made special mirrors, attached them to balloons and watched the sunbeams on the plane, which significantly raised the sensitivity of the device. By doing so he succeeded to sufficiently establish the exact size and find the value of the mass of the Earth.
 Foucault's experiment
Foucault's experiment
Although the KP wrote about it before, I’ll repeat that the French experimentalist Jean Bernard Foucault in 1851 proposed an experiment that demonstrated the Earth's daily rotation. Based on the assumption that during its oscillating motion the plane of the physical pendulum remains in the frame of reference, which is related to the stars, fixed, by using the 67-meter pendulum suspended under the dome of the Pantheon of Paris, he managed to demonstrate to an observer, who is standing on the Earth and rotating with it that the plane of vibrations is gradually moving away from its original position in the direction opposite the direction of rotation of the Earth. Since that time such pendulums, which generally were called Foucault pendulums, were built in many countries, and one of them - in the KPI.
Experiment of Isaac Newton
In 1672, Newton made a simple observation. After darkening the room, he made a hole so that the sunbeam was visible there, had a well-defined shape, and put a glass prism, behind which was the screen on his way. As a result, there was a rainbow on the screen, indicating the conversion of so-called white or solar, light on several colourful - from red to purple. Such schedule of the white beam of light on several others was called dispersion.
With a series of experiments with crossed prisms Newton showed that white light is a composite, and components are all colours from red to violet.
 The experiment of Thomas Young
The experiment of Thomas Young
For centuries, until the XVII, existed the ideas that light is nothing but a stream of single particles - so-called corpuscles. Although the phenomenon of diffraction and interference observed even Newton, the view of the corpuscular nature of light was still common.
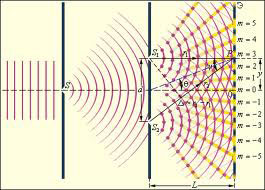
However, for the waves was a well-known phenomenon of interference, which is about periodical amplifying or diminishing the amplitude of the oscillation of two waves that exist simultaneously in space. English physicist Jung in 1801 decided to test whether phenomenon of interference is inherent in light. For this, he began experimenting with his beam, which was directed on opaque plane with two slits. As a result, on the following screen behind the plane the researcher rather unexpectedly for the first time observed a light interference pattern consisting of alternating light and dark bands and could not be created by the flow of particles. Dark streaks was suitable to the zones of mutual quenching waves from various sources-gaps and light – to the zones of their (waves) addition. Thus, Jung was able to conclusively prove the waving nature of light.
Experiment of Claus Jonson
This research was made by the German experimental physicist relatively recently, in 1961, when Young’s measurement, was played back, but for real particles. Johnson, passing electrons through two slits, also observed a pattern, similar to that Jung had seen, which clearly indicated the benefit of quantum mechanics that avers wave-particle duality, or mixed nature of elementary particles.
 The experiment of Robert Millikan
The experiment of Robert Millikan
This experiment was carried out in 1913 and concerned the nature of electric charge – whther it is discrete or continuous. In the physical usage was introduced the word electron that meant some particles - elementary electric charge carrier. However, this definition remained formal, since neither the particle nor attributed to her elementary electric charge was not known. At the turn of the nineteenth and twentieth centuries, and specifically - in 1895, the German physicist Conrad Roentgen discovered that bit tubes anodes under the influence of falling on them rays from the cathodes generate some radiation, which we call the X-ray. That same year, Frenchman Jean Baptiste Perrin was able to experimentally prove that cathode rays is nothing but a stream of unknown negatively charged particles. However, despite the availability of experimental evidence on such particles, the electron was a hypothetical object.
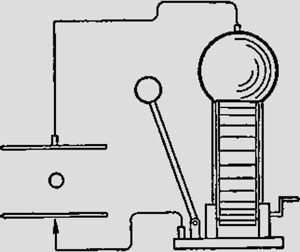
American experimentalist Robert Millikan developed a method of drops by which he learned how to isolate in the interval between the capacitor plates relatively small number of charged liquid drops. Further by the action on the air between the plates of X-rays, it could be ionized, that changed the charge of drops.When the capacitor was charged, the electric field could induce and observe the movement upward of suspended droplets, and when the capacitor was excluded, they moved down. He started experimenting with drops in 1906 and continued to do this with the utmost care for several years. By 1909-1910 with great accuracy he persuaded that drops charges vary only discretely, and change is always a multiple of some fundamental value e that was matched with electron charge. The results at first proved a discrete nature of the charge, and electrons were actually existing particles. The first measured value claimed that module e ~ 4,89 x10-10 electrostatic units, or CGSE.
Without any exaggeration Millikan’s research began milestone in the development of physics in the twentieth century, and in 1923 he was awarded with the Nobel Prize for them. Now, the exact value of the elementary charge of an electron is considered to be the value of e ~ 4,8032 x 10-10 CGSE.
 Ernest Rutherford’s Experiment
Ernest Rutherford’s Experiment
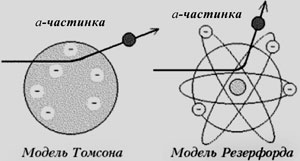
The beginning of the twentieth century was marked by rapid development of different areas of physics, in the asset which was not only an understanding of the atomic structure of matter, but also some knowledge about the structure of atoms themselves. They were considered indivisible and those that are composed of two kinds of particles - negatively charged electrons and positively charged of unknown nature, so that in total atoms are neutral entities. Such an ideas of the positive-negative and essentially mixed nature of the charge distribution in the nuclear system were at that time very common, but there was no experimental evidence about its spatial density, or the actual size of atoms. The vast majority of physicists shared the view of the famous English physicist Joseph Thompson, who in 1903 proposed a model of atomic structure. For her thoughts, the atom is a positively charged sphere that has a diameter of about 10.8 cm, with the floating inside electrons.
This continued until 1909, when, perhaps, the first nuclear physicist Rutherford decided to check, and what actually the structure of the atom is. To do this, he used opened himself relatively heavy and positively charged a-particles (their charge ea = 4e), which accelerated to a speed of 20 km / s and directed at a thin gold foil, scattering in it on the atoms of gold that indicated their (particles) deviation from the original direction. It was measured by flashes on the scintillator plate that were conditioned by falling on it scattered a-particles. In these measurements, Rutherford watched a lot of flashes and proved that one of on average nearly 10,000 a-particles deflected at an angle greater than 90o. The last fact indicated that in such acts a scattering particle actually turns back, which could not occur if the atom was similar to the neutral structure of Thompson.
After analyzing the results, Rutherford stopped on the model that best described the received data. Thus, he proposed the planetary model of the atom that in a way was reminiscent of the heliocentric. In the planetary model of the atom proposed by Rutherford, atom is a tiny massive nucleus with the size not exceeding 10-13 cm, with electrons orbiting around it on orbits of radius 10.8 cm
This is how modern experts have seen the most significant experiments of the past, which caused our progress on the difficult path to a correct knowledge, correct views on the universe and the nature of things.
And the last thing that is worth saying, thinking of readers Kyiv Polytechnic and students of Science Faculties of KPI. We must firmly know that physics is the science of young, so it is better to start engage in physics, show interest in the world as soon as possible. Rules in physics, in general, are complex, and master them can and should only through hard, honest work, which is also better to start at a young age when you are full of strength, and your memory is developed.