Biomedical Engineering (nextly BME) - examines and develops technical and artificial biological objects, as well as medical equipment and technology of production and maintenance, quality control and certification for safe use in all areas of biology and medicine, explores their interaction with life forms.
This interdisciplinary branch of education which combines engineering and life sciences, covering a wide range of activities from direct clinical use to long-term, basic research of natural and synthetic biomedical objects.
BME occurred at the intersection of exact and biomedical sciences as a formal academic discipline in the early 1950s, although the interpenetration of engineering knowledge and science in biology and medicine began much earlier.
Direction of Biomedical Engineering launched doctors, that attached leading technical universities to solve medical problems. The first institution where it happened: Institute of Medical Physics (1921), further - Biophysics Max Planck Institute (Germany) and Radio of Engineering Institute (1948, USA). In the future, most of the leading technical universities and faculties opened Department of BME. Some are led by doctors, such as Professor of Physiology L.Heddes (Purdue University, USA), Professor of Medicine T.Harris (Vanderbilt University, USA). It were organized national and international professional associations that have joined researchers, teachers, engineers and doctors of the world in BME. Thus, in Europe and the United States formed a powerful movement to create an independent training programs of BME, which is no longer part of traditional engineering programs, and initially fully integrated with medicine and biology.
Today the development of materials medical products, their production and medical technology is one of the main directions of economic policy of developed countries. The implementation of these programs needs to attract highly educated professionals who have the appropriate amount of knowledge and skills in the branches of biology, medicine HN, medical equipment, medical electronics, biomaterials. This led to the creation in the US and Europe "Biomedical Engineering" and related to it specializations (such as, "Medical Informatics", "Medical Engineering", "Clinical Engineering").
According to United States Department of Labor, from 2000 to 2010 the number of working places in BME increased by 31.4% that twice the rate of growth exceeds employment in all other areas.
Total in the world by 2000 revenues associated with medical technology and medical equipment, reached 160 billion euro, of which 41.5% received in the US market, 25.6% in the EU, 15% - in Japan.
Turnover of capital in this area has increased continually, creating a powerful market of medical materials, equipment, technologies and therefore working places. The rapid growth of production led to an increase in the number of items of medical equipment (which is made only in Europe 12000 companies) from 840 thousand in 1994 to 1400 thousand in 2004.
In 2000 only sector of development and manufacture of medical devices in the EU employed 315,000 professionals, not including staff involved in the operation and maintenance of medical equipment in health care institutions. In 2001 one of the largest multinational companies - Siemens, which consists of 13 competitive industrial sector received more revenue from medical devices than from any other goods and services (excluding the energy sector).
Overall, BME sector occupies a prominent place on the effect on the European economy. BME will require strenuous educational efforts to provide it with the necessary human resources. It is also associated with a constant rate proportional increase in biomedical engineers need in the countries which recently joined the EU or are planning to sign in the near future.
Unification of quality and accreditation of educational programs followed the rapid spread, and political changes in Europe that increase the ability and mobility of employment in the European Higher Education in need of universities and professional associations in the field of BME active participation in shaping the future of this very successful and promising management disciplines through the development of higher education and research in this field.
More than 150 universities, polytechnic schools and academies in Europe offer educational programs on science medical bioengineering all academic levels that require international coordination and content requirements for initial training.
To further support a high quality of biomedical engineering education created "International Federation of Medical and Biological Engineering", and supported its newly established "European Alliance Medical and Biological Engineering and Science" that brings together national and multinational European associations, educational institutions and industry, associated with medical and biological engineering and science. These organizations keep records and coordination of all medical biological educational and research programs in Europe and prepare recommendations to create a competitive and harmonized education accreditation of a large number of different programs for training of medical and bioengineering experts.
According to this, the 2005 International Federation of Medical and Biological Engineering approved "Criteria for Accreditation of Educational Programs BMI for Europe" which are suitable to the requirements of the Bologna Declaration. The main participants in the process are universities, because European University Association identifies important areas of the European Higher Education. These guidelines apply to BME.
Priorities in this context are:
• accreditation of educational programs;
• training;
• continuing education throughout their lives.
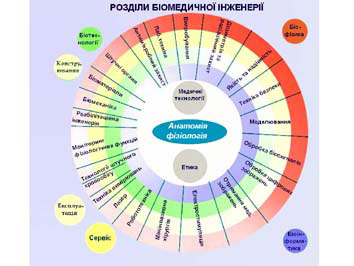
Achieving employment opportunities unimpeded in Europe and sharing new technologies requires harmonization of higher education in the field of BMI. With this in mind, accreditation of educational programs with BMI, based on criteria agreed upon in Europe, is a prerequisite for the realization of all other tasks. The real problem is the initiation of European institutions for accreditation, comparison and mutual recognition of educational levels of BMI, which would work on the basis of international agreement. In order to develop recommendations on Working Group for Accreditation and Certification of the German Society for Biomedical Engineering (DGBMT) took the initiative Cataloguing key definitions. As a result of this work created the document has 50 pages, which is almost resulted port BME specialty.
The said working group identifies areas of BME are given in the diagram.
Given the diversity of scientific fields and BME training programs that now exist in Europe, their accreditation rules require dynamic research and rules.
Accreditation provides BME as it is and also mixed education at Bachelor's programme, master, doctoral, and programs that consist of one or two degrees, training and continuing education.
While preparing the accreditation criteria originally came from the common situation where the BME was integrated into the traditional engineering education in the curriculum laid flexibility and breadth that allow you to adapt these criteria to a sharp increase in the number of programs BME, completely independent from traditional engineering training programs. Of course, for such a dynamic discipline, which is the BME, the criteria for accreditation are continuously reviewed and adapted to frequent renovation of educational programs.
Required for educational direction BME knowledge and skills can be grouped into the following categories:
1) basic sciences and engineering disciplines, including engineering design technology, construction, design, production;
2) general knowledge and important skills such as the ability to work in a multidisciplinary team of specialists, technology management, business and economics, quality control, professional ethics, creative thinking, understanding of cultural, social, economic and political impact of technology;
3) biomedical engineering knowledge, skills and abilities required for qualification.
The nature and fast developing field of biomedical engineering, and as a result is a variety of types professional needs to determine the levels of competence, for example such as the widely used - technician, engineer, specialist, master's degree, PhD, or the language of the future European structures: single, two, three degree programs.
Despite the level of the minimum amount of competence on the foundations of biomedical disciplines should include: anatomy, physiology, medical terminology, biochemistry, cell biology and genetics.
The amount should cover general subjects such as: foreign language; didactics, technology presentations; project performance; work in a team; fundamentals of economics and law; Interdisciplinary work; process control; economic and business administration; quality control; professional and medical ethics; cultural, social, economic and political effects of technology.
Also required extensive knowledge of biomedical engineering, clinical engineering, medical physics and medical science.
Each of these four specialties include four major items.
Biomedical (and medical) Engineering:
- Medical Electronics and monitoring;
- Medical image;
- Biomaterials;
- Biomechanics.
Clinical engineering:
- Hygiene;
- Laboratory and analytical equipment;
- Safety and quality;
- Medical Electronics, and monitoring.
Biomedical informatics:
- Processing of digital images and computer graphics;
- Communication and Information Systems;
- Biomedical Statistics;
- Processing of biosignals.
Medical Physics:
- Protection against radiation;
- Dosimetry and radiotherapy planning;
- Medical image;
- Modeling and simulation.
List of basic knowledge Bachelor BME and clinical engineering follows:
Biomedical engineering:
- Biomedical equipment and technology, biometrics, bioelectronics, medical electronics and monitoring, receiving and processing of biosignals and medical imaging, biomaterials and biocompatibility, service and technology operation of medical equipment, biomechanics, telemedicine, modeling and simulation, rehabilitation engineering, construction and design, computer graphics, medical informatics, cell and tissue engineering, laboratory equipment and analytical aspects of legalization (certification) of medical products.
Clinical Engineering (optional):
- Hygiene, laboratory and analytical equipment, quality assurance and safety aspects of legalization (certification) medical products, radiation protection, information and communication systems of hospitals, statistical methods in medicine.
Depending on the professional direction these items selectively depth study, supplemented by selective study: artificial organs and artificial circulation technologies, active and passive implants, artificial ventilation equipment and anesthesia, use of computers and information technology in medicine, information systems, information management, lasers in medicine, planning and the use of radiation therapy, BME in radiation medicine, radiation and radiation protection, endoscopes, mini-invasive surgery, computer add-surgical technology, medical robots and manipulators, biocompatibility of materials and surfaces, prostheses, medical, measuring and optical instruments , micro- and nanotechnology.
The curriculum of BME in Ukraine, as in Europe, should provide education that allows you to organize and carry out fundamental and applied research in the multidisciplinary field that brings together the scope of engineering sciences, biology and medicine. The specialists must have experience of an integrated approach to the object, which allows you to: develop existing interdisciplinary concepts and create new ones; to participate directly in the development of high-tech approaches to intervention in life processes, monitor their effectiveness. BMI is the link that makes the relationship of clinical practice, research and production. Biomedical engineering specialists have a dual function: on the one hand, they introduce scientific advances into clinical practice, and the other - transforming the needs and objectives of clinical practice in the areas of research and production. Because this interaction creates new tools and technologies for medical practices, bachelor specialty "Biomedical engineering" can be completed specialization in medical engineering, clinical engineering, biomedical physics, biomedical informatics, computer science and information technology, as well as already existing in Ukraine specializations such as physical and biomedical electronics, medical devices and systems, biotechnical and medical devices and systems, medical acoustic and bioacoustics instruments and devices and others.
Because of the mentioned areas of clinical training of medical and engineering are new for Ukraine applied components BME should be emphasized that they are aimed at ensuring compliance technology use in medical practice sophisticated equipment, use of materials and medical devices; control and certification of their quality and safety; accuracy in obtaining, processing and interpretation of biomedical information. Special education work involves engineering staff directly to the medical and research institutions of the health system. Medical engineers must be ready to work together with the medical person scrap the areas of application, interpretation of results and efficiency technologies tehnoyemnyh diagnosis and treatment.
It should be noted also that the inclusion Ukrainian field of study of BME to the field of knowledge "Biotechnology" meets the latest world trends as medical biotechnology, associated with the use of molecular chemistry (genetic engineering), stem cells, cell and tissue engineering, there is a section of modern BME.
Recently, experts from Europe BME (such as medical engineering), having additional secondary medical education, are directly involved in the application tehnoyemnyh methods of diagnosis and treatment. For example, magnetic resonance imaging, and auxiliary artificial circulation, programming and adaptation of the artificial heart rate of drivers and more. It is also an important strategic direction of BME education.
In Ukraine, the production of medical equipment and medical supplies very confined-driven and is at the initial stage of development. Providing all the needs of national health care in the above products and technologies through imports unnecessary, and sometimes impossible for economic reasons. It is necessary of domestic-established logistical and scientific base for the production of medical equipment, materials and means of prevention. In addition, implementation and operation of high-tech medical equipment and specialized medical supplies puts forward new requirements for the competence of experts, developers and engineering staff that accompanies the installation and operation of equipment in hospitals.
The crucial problem in the development of advanced medical technology and medical technology is the need to overcome the main limiting factor, namely the lack of specialists in BME.
Along with this, it would be appropriate, given the experience of higher technical educational institutions of Ukraine, to combine different areas of specialization at the undergraduate, followed by a single formation master's degree level training, the experience, expertise and logistical framework graduating department.
Thus, given the European experience and trends of Bologna process, one could argue that the development of BME in Ukraine is the strategic direction of strengthening the state's economy, improving the efficiency of domestic production of medical and health care, opening a principally new field of research.

