On September 22nd was 225 years since the birth of the great English scientist Michael Faraday (September 22, 1791 - August 25, 1867), two discoveries which lie at the base of electroenergy: in 1821 he created the prototype of the electric motor, and in 1831 discovered the phenomenon of electromagnetic induction, which lies at the operation of electric generators. Mastered the Faraday views on the nature of electromagnetic phenomena and using the results of his experiments, James Clerk Maxwell created his own theory of electromagnetism. Faraday also received a number of other prominent scientific results, in particular discovered and investigated the phenomenon of paramagnetism, diamagnetism, rotate the plane of polarized light in a magnetic field, discovered the laws of electrolysis.
Michael Faraday studied only in elementary school, where mastered the skills of reading, writing and arithmetic. All other knowledge and skills necessary for research in physics and chemistry, he earned himself using all the opportunities that were opened before him.
It should be said that the first of these opportunities was extremely small. Michael Faraday was born in London, in the family of a blacksmith James Faraday, who worked hard to make bread. Childhood and adolescence of Michael occurred in the period of the French Revolution and subsequent European wars that have involved England. War adversely affect the British economy, which suffered primarily the poor, to which belonged Faraday`s family.
At the age of 13, Michael was sent as a student in the bindery at a bookshop that belonged to emigrant Frenchman Ribot. At the first year, Faraday passed the probationary period, working as a newsboy, and then, under a contract that signed between his father and Ribot, he had been taught for seven years without pay – for food and shelter. At first, Faraday have difficulties, but he tried, and in the fourth year of study, he actually mastered the craft of a bookbinder.
Undoubtedly, such work helped self-education of inquisitive boy, who from childhood was addicted to reading. In those days released printing books were without bindings (and uncuted), but buyers of the books worried by themselves about binding. Thus, through the Faraday`s hands were passed the latest books, including scientific.
 Apparently, crucial to further fate of Michael that in 1805 to his hands came the book of outstanding popularizer of science of XIX century Jane Marcet (Jane Marcet, 1769-1858) "Conversations about the Chemistry". This book was the first in the world popular science book that has an immerse success. Within 50 years in England published 16 editions of the book, in France – three in the US – 23, with total circulation of 160,000 copies. Such success was not accidental. The book is written very accessible in the form of dialogue, teachers with two pupils, one of which – serious and understanding everything on the spot and the second – frivolous, thus to her the material explained in more and more accessible form.
Apparently, crucial to further fate of Michael that in 1805 to his hands came the book of outstanding popularizer of science of XIX century Jane Marcet (Jane Marcet, 1769-1858) "Conversations about the Chemistry". This book was the first in the world popular science book that has an immerse success. Within 50 years in England published 16 editions of the book, in France – three in the US – 23, with total circulation of 160,000 copies. Such success was not accidental. The book is written very accessible in the form of dialogue, teachers with two pupils, one of which – serious and understanding everything on the spot and the second – frivolous, thus to her the material explained in more and more accessible form.
The book consists of two volumes containing 10 and 14 sections. The first section gives a definition of chemistry as the science of simple body (i.e. chemical elements) and their interaction, is cited classifications of simple bodiesto which in the early nineteenth century attributed the light, heat and electricity. The second section describes submitted instruments for the study of chemical phenomena. The third and fourth sections dealt with the light, heat and thermal capacity bodies. The fifth section devotes to chemical sources of current (galvanic cells and batteries). In chapters 6-10 mentioned about the properties and methods of simple substances, oxygen, nitrogen, hydrogen, sulfur, phosphorus, carbon and metals. The second volume begins with a section which described the conditions necessary for the interaction between simple substances. Then, the sections, which dealt with the properties of alkali, metal oxides, acids, organic substances.
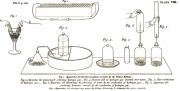
Especially valuable in this manual was that it contained descriptions of experiments, illustrated with drawings of the author. In the descriptions give the smallest details of the experiments that base on the necessity of thorough preparation and contained warnings about possible dangers. This book has become very popular as a guide to conduct experiments, lectures in schools. Incidentally, after the book about chemistry Jane Marcet has issued 31 non-fiction books, which wrote about physics, mineralogy, political economy, botany, philology, geography, history and others. Many of her books are available in electronic library Internet Archive (https://archive.org/), where everybody can read material.
In 1858 in a letter to the Swiss physicist Auguste Delyaria, Faraday described his impressions about Marcet`s book: "I felt then that I have found resistance of its chemical knowledge, and firmly grabbed for it. Hence the reason for my deep respect for Ms. Marcet. Firstly, it brought me personally great joy and made a real blessing, and secondly, she was able to open to young, ignorant, inquisitive mind the phenomena and laws of the immense world of natural science. You can imagine my delight when I personally got acquainted with Ms. Marcet. How often did I mention the past and compared it with the present, so often I thought about my first teacher and always believed it is a duty to send their work as an expression of gratitude. And these feelings will never leave me."
In the same letter, Faraday wrote: "Do not think, please, that I was a deep thinker or was different in early development. I was a moving boy with a rich imagination. I believed in "Thousand and one night ", as much as in "Encyclopedia". But for me there were important facts, and it helped me. The fact I could trust, while each statement I could always oppose another statement. Therefore, I checked Ms. Marcet`s book, performing these simple experiments to conduct which had the money and then saw that book accordance with the facts as I understand them".
It is noteworthy that produced during adolescence habit to rely solely on the facts and check statements by other own experiments, Faraday kept for the whole life, due to which he made many discoveries.
But deal with experiments the young student-binder was very difficult. He worked without payment and had only casual earnings for working overtime and on weekends. From his experiments Faraday could literally spend a penny. Electric car made from a bottle. Having bought a few pence and zinc plate instead of using copper plates pence coins, made galvanic battery.
As a child Faraday has another good habit. At the age of 9 he began to record interesting information in a notebook, which then put together to a handwritten book called "Philosophy collection of various articles, notes, events, accidents and so on that related to the arts and sciences and gathered from newspapers, reviews, magazines and other works to promote satisfaction, self-learning and as confirmation and refutation of the theories prevalent in the scientific world. Compiled by Michael Faraday from 1800 to 1809 ".
Faraday's self-education was helped by the fact that at the beginning of the XIX century, British scientists began to read paid public lectures from various sciences. In 1810 Faraday learned from the announcement that Mr. Teytum will read in his own house lectures on natural science and entrance fee was one shilling. Faraday`s boss allowed to attend lectures, and the money gave elder brother Robert, who has worked as a blacksmith. From February 1810 to September 1811, Faraday listened thirteen lectures and carefully took notes.
On these lectures Faraday met and became friends with some of the students who decided to form a group of self-education and self-improvement. At meetings of this group Faraday told about conducted by him experiments or books that he had read, moreover the members of the group discussed not only the substance that was set out, but also a form of presentation. The comrades carefully indicated all the mistakes and future he tried to avoid it. Subsequently to improve speech he took lessons in oratory.
With some of the members of the group Faraday kept close relations and corresponded until his death. Most of all he wrote to B.Abbott, R.Phillips. The last subsequently became a member of the Royal Society. The first letters to friends Faraday wrote, trying to learn to express their thoughts in written form and to develop satisfactory writing style.
Faraday's self-education has contributed tenants of the owner – French emigre artist Masker. Michael cleaned his room and cleaned shoes. For this young bookbinder Masker taught to draw and paint.
 In 1813, a member of the Chartered Institute, William Dance ordered binding for journals in chemistry. Inspired by reading them, Faraday has delayed the order that caused the complaints of the customer. But when Dance learned about the cause of the delay and saw how serious this binder studying chemical journals, he touched and invited him to present in one of the books of their choice. Faraday has chosen the book one of the founders of electrochemistry Humphrey Davy. Then Dance gave him an invitation to the four public lectures of Humphrey Davy, which determined the Faraday`s entire fate.
In 1813, a member of the Chartered Institute, William Dance ordered binding for journals in chemistry. Inspired by reading them, Faraday has delayed the order that caused the complaints of the customer. But when Dance learned about the cause of the delay and saw how serious this binder studying chemical journals, he touched and invited him to present in one of the books of their choice. Faraday has chosen the book one of the founders of electrochemistry Humphrey Davy. Then Dance gave him an invitation to the four public lectures of Humphrey Davy, which determined the Faraday`s entire fate.
At that times Humphry Davy was the most famous British chemist. He performed one of the first electrolysis of water and identified it as a compound of hydrogen and oxygen. Through electrolysis of some alkalis, which then were considered simple substances, in 1807-1808 he opened several metals – potassium, sodium, barium, calcium, magnesium, strontium. In 1808-1809 he discovered and described an electric arc. One of the first he got Bor and proved that Chlorine is a simple substance, but not Oxide. Davy also defined the role of minerals for vital functions of plants and was a founder of agricultural chemistry. April 8, 1812 for scientific achievements he received the title of knight of the British Crown, and two days later married a rich young widow aristocrat Jane Eypris. Davy also was famous for his public lectures on chemistry and agricultural chemistry, who collected hundreds of students.
Faraday recalled: "When I was an apprentice, I was lucky enough to hear the last four lectures of Sir Humphry Davy... I made a short recording of lectures, and then rewrite it thoroughly and illustrated by such figures which I could make. The desire to engage in scientific work, even +most primitive prompted me, a novice, unfamiliar with the secular rules, to write a letter to Sir Joseph Banks – to President of the Royal Society. Logically after a while I find out that my request left unanswered".
Meanwhile, on October 7, 1812 has expired Faraday`s study. The next day he began working in the bindery of another French emigrant Delyarosh. Unlike Ribot, the new owner was tough and arrogant and soon to work at him became quite unbearable. To Faraday this led to act decisively – he wrote Devy about the desire to engage in research work by adding an evidence of seriousness of their intentions by recording of his four lectures. The answer came immediately – friendly, but negative. The institute had no vacancies. But soon, during one of his experiments in a laboratory the flask exploded, its shrapnel injured Devy`s eyes, and for a while he could neither read nor write. Remembering about bookbinder who was interested in science, Davy decided to take him to himself until recovery as the secretary that at the same time to make acquaintance with him.
A few days later Davy eyes healed and he parted with Faraday. But this time, he had a good impression on Davy by his knowledge and diligence. When in a few weeks in the Davy`s lab released laboratory assistant vacant seat, he offered it Faraday.
In the protocol of the Chartered Institute from the March 1, 1813 has a record: "Sir Humphry Davy is pleased to inform the directors who found a man that is desirable to appoint on the position... his name - Michael Faraday ... His information seems to be good, his character is active and cheerful and has a reasonable way of action".
Besides working, Faraday got an apartment with two rooms in the building of institution. Prior to his duties was to keep order in the laboratory, to submit all the necessary devices to Devi during the experiments, subjects bring in the audience for public readings all necessary materials and devices, and then to carry them back.
Thanks to the Davy patronage Faraday, in addition to his duties, was able to start research. In particular, with the permission of Davy he continued unfinished experiments, because of the risk with those experiments with nitrogen chloride – extremely explosive. Faraday`s experiments brought to the end, though their conduct during four strong explosions occurred at one of which he was seriously wounded fingers of one hand, and his face remained unharmed because he worked in a glass mask.
At the same time, Faraday took an opportunity to attend lectures and reports that pronounced in the Royal Institute, thus constantly filled up his knowledge. He also analyzed the impressions of listened lectures and tried to explain to himself why these lectures sometimes were interesting and sometimes not. This helped him to develop proper methods of lecturing and later he was famous as an excellent lecturer. By the way, the Internet has a very interesting article: R. Syher "Michael Faraday and Arts of reading lectures" (Successes of physical sciences. 1970. T.100. Part 1. P.147-162).
 A few months after Faraday started working in the Royal Institute, Humphry Davy with his wife went on a journey across Europe. He took with him on the trip a portable chemical laboratory and invited Faraday to be his assistant and secretary. A few days before the trip Devy`s servant refused to go, so he offered Faraday temporarily – until he found another – to perform the servant duties.
A few months after Faraday started working in the Royal Institute, Humphry Davy with his wife went on a journey across Europe. He took with him on the trip a portable chemical laboratory and invited Faraday to be his assistant and secretary. A few days before the trip Devy`s servant refused to go, so he offered Faraday temporarily – until he found another – to perform the servant duties.
During the trip Faraday kept a diary, in which recorded the all the impressions and opinions. This diary gives an idea of how much Faraday has discovered on this trip. After all, he spent 22 years in London, and when traveled, it was not further 12 miles. But with Humphry Davy, Faraday visited France, Italy, Switzerland and other countries.
In Paris Devi met and discussed the scientific issues with Jean-L.Hey-Lussac, Alexander von Humboldt, K.L.Bertole and other famous scientists. And not only discussed, but also conducted some research. In particular, following the analysis of the ash seaweed, containing iodine, Davy concluded that iodine – a chemical element similar to chlorine. With this conclusion, Gay-Lussac strongly disagreed, but two years later, after many experiments admitted Davy`s acknowledge correctness.
In Genoa Faraday helped Davy to conduct experiments with electric ray, which purpose was to find out whether electrical discharge causes decomposition of water by devil-fish.
In Florence Davey and his assistant spent burning the adamant in an oxygen atmosphere, and finally brought the common nature of adamant and graphite. However, Devy took the largest unique lens, which belonged to the Grand Duke of Tuscany. With its help together Davy with Faraday directed rays of the sun on the adamant, which lay in a platinum cup under the glass case filled with oxygen. In Florence Faraday and Davy visited the telescope made by Galileo.
In the Faraday`s diary has a record: "Friday, June 17, 1814, Milan. I saw Volt, who came to Sir H. Davy: he is cheerful old man, on his chest – red tape, very easy in conversation".
In Geneva, Davy and Faraday became acquainted with the doctor and physicist Charles Delyariv and his son Auguste, who was at that time only 13 years old. Six years later, in summer 1820, Auguste Delyariv will show to Fransua Araho experiment of Esterd with the impact of electric current on a magnetic needle. In September, Arago`s research will show that at the meeting of the Paris Academy of Sciences, Academician Alexander after which A.-M.Amper, Gay-Lussac, J.Bio, F.Savar and other, began to research electric and magnetic phenomena.
It is hard to overestimate the role of this trip for the formation of Faraday as a researcher. Davy was a great scientist, and support in his experiments was an excellent school for the experimenter and just presence at the talks of great scientists an attentive and curious young man could replace a bunch of books and put it in the latest range of scientific problems.
After returning in 1815 to London, Faraday once again began work in the chemical laboratory of the Royal Institute. He took an active part in preparing lectures on chemistry and physics, as well as various kinds of tests and other work performed in the chemical laboratory. His salary has raised. In 1816 the magazine "Quarterly Journal of Science", which was published by the Royal Institute, published the first printed Faraday`s work. This happened with a Davy`s light hand: he instructed Faraday to perform chemical analysis of Tuscan lime from Italy, and when Faraday gave results to Davy, he gave it for publication in the journal.
From 1816 to 1820 Faraday performed various research in chemistry and physics and published its results in the Journal of the Royal Institute. He won among colleagues such authority that in 1819 he was assigned to edit the magazine. In 1820 he published the work "On two new compounds of chlorine and carbon, and the new compound of iodine, carbon and hydrogen." This work has such high level that it was read at a meeting of the Royal Society and published in the Journal of association "Philosophical Transactions".
However, all this research as it turned out later, were only for Faraday steps to its main scientific achievements that began after 1820 and opened a new era in the history of physics and engineering.


